It’s been a few months since I posted the handout version of my Quantified Self talk analyzing a little over a year’s worth of my blood glucose data from my Dexcom continuous glucose monitor. I kept meaning to write out a long-form version of my talk, since the handout/slides don’t contain much exposition, and now I’m finally getting around to it.
Introduction
Type 1 Diabetes
(You can skip this if you’re a PWD. If you’re just interested in my visualizations of Dexcom data, you can jump to that section.)
Type 1 diabetes is an autoimmune disease (like lupus and rheumatoid arthritis). In type 1 diabetes, the body’s immune system attacks and kills the insulin-producing β-cells in the pancreas. This results in very little to no endogenous insulin production, which means that the person with diabetes (PWD) is dependent on synthetic insulin.
Today’s synthetic insulins are produced via genetically-modified E. coli bacteria. The resultant biosynthetic insulin is delivered by injection or infusion (with an insulin pump) into the subcutaneous fat layer. It’s important to note that this method of delivering the drug does not very effectively mimic the production of insulin in a non-PWD. Injection into subcutaneous fat is not a very fast method of drug delivery: even the fastest of the modern “fast-acting” insulin analogs (NovoLog, Humalog, Apidra) don’t reach their peak action until around 45-90 minutes after they are introduced into the body.
Insulin Dosing
Insulin dosing is more of an art than a science. (Please excuse the total cliché.)
There are two types of insulin doses that everyone who doesn’t produce their own (or enough of their own) insulin needs to survive and thrive: basal insulin and bolus insulin.
Basal Insulin
Basal insulin is the “background” insulin—the insulin needed by the human body just to carry out normal functioning. PWDs who use an insulin pump get their basal insulin via a constant, slow drip of insulin infused through the cannula that is embedded under the skin in the subcutaneous fat and that is the outlet of the infusion line connected to the reservoir of insulin inside the pump. PWDs who don’t use an insulin pump take a once-daily injection of a delayed-release “long-acting” insulin (e.g., Lantus or Levemir).
Bolus Insulin
Bolus insulin is the insulin that a PWD takes on an as-needed basis to “cover” carbohydrates ingested as well as to bring down high blood glucose. PWDs who use an insulin pump can deliver a bolus of insulin at any time using the pump’s controls. PWDs who don’t use an insulin pump will inject bolus insulin using either syringes and a vial of insulin or an insulin injector pen, a device with a helpful dial mechanism for drawing up precise doses of insulin.
When a PWD is taking insulin to cover carbohydrates s/he is intending to consume, s/he calculates how much insulin to take using an insulin-to-carb ratio—that is, a ratio defining how many units of insulin can be expected to cover a certain number of grams of carbohydrate. There is no universal insulin-to-carb ratio. Rather, every PWD must determine the insulin-to-carb ratio that works best, and this may even vary by time of day: many PWDs find that they need a larger insulin-to-carb ratio (i.e., more insulin required to cover fewer grams of carbohydrate) in the morning.
My Type 1 Diabetes
I was diagnosed with type 1 diabetes in December of 2003 at age 19. I was quite lucky in that my diabetes was diagnosed without my going into diabetic ketoacidosis (DKA), a dangerous condition (sometimes resulting in coma or death) that arises from prolonged very high blood sugar and little or no insulin in the bloodstream. Perhaps because I was diagnosed before I was at the dangerous point where I wasn’t producing a significant amount of my own insulin, my type 1 diabetes has always been somewhat mild, in comparison to others, likely because I am still producing small amounts of my own insulin. (At least as of November of 2006, this was confirmed by a non-zero result with a C-peptide assay. C-peptide is a by-product of insulin production, so a non-zero C-peptide implies non-zero insulin production.)
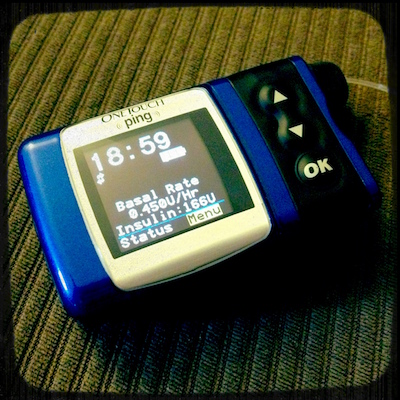
My Insulin Pump(s)
Since December of 2006, I have been using an insulin pump. I started with a Medtronic Paradigm 522, but since January of 2011 I’ve been using an Animas/OneTouch Ping pump.
Continuous Glucose Monitoring
I started using a Dexcom continuous glucose monitor in February of 2011. The Dexcom system involves a sensor wire embedded under the skin in the PWD’s subcutaneous fat tissue. A transmitter snaps into the sensor wire’s cradle and transmits a blood glucose reading to a separate, wireless receiver unit every five minutes.
Some visual aids:

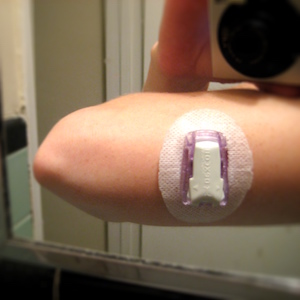
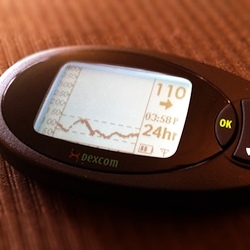
Features of the Dexcom
As well as providing an (approximate) blood glucose reading every five minutes, the Dexcom system includes several other useful features. There are seven possible different trend arrows that indicate the rate at which the PWD’s blood glucose is changing: a steady arrow, three speeds of up arrow, and three speeds of down arrow.

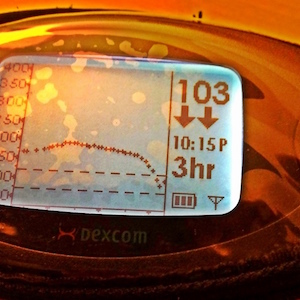
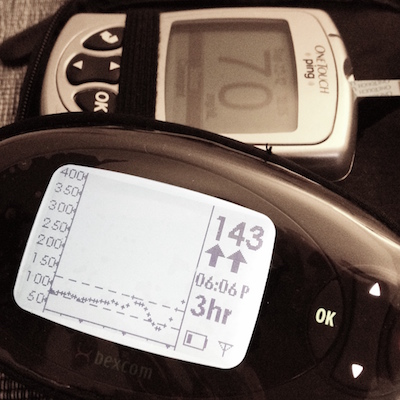
The Dexcom is also user-programmable to provide audible and/or vibrating alerts when the PWD’s blood glucose is above a certain threshold, below a certain threshold (and, for safety reasons, below a hard set 55 mg/dL), or changing very rapidly. The Dexcom system also comes with Windows-only software that allows the user to download his/her data in basic XML or tab-delimited formats.
Accuracy of the Dexcom
The main utility of the Dexcom system is not to replace fingerstick blood glucose monitoring but rather to provide a fuller picture of a PWD’s blood glucose fluctuations. PWDs who use a Dexcom system are encouraged to continue fingerstick testing whenever making a treatment decision: at minimum, this means fingerstick testing before every meal (in order to determine the proper insulin dose), before exercise, and before going to sleep. In addition, the Dexcom system requires calibration with a fingerstick test approximately every twelve hours.
The Dexcom sensor wires are designed (and approved by the FDA) to be used for seven days, but many Dexcom users (including me!) will “reboot” a sensor after the initial seven days have passed and continue to wear the same sensor wire for up to 14 days. Accuracy of the system is greatly improved by doing this since the sensor is working from a greater number of calibration readings.

A corollary to this is that the accuracy of a sensor is often particularly low on the first day or two after the PWD has inserted a brand new sensor wire. Whenever a fingerstick reading differs by more than 20% from the Dexcom’s reading (as in the picture to the left), entering the fingerstick reading as an additional calibration is recommended.
My Experiment
Introduction
Blood Glucose Management
Blood glucose levels in a non-PWD vary between 70–130 mg/dL and only reach the higher end of that range after high-carbohydrate meals.
Many PWDs aim for a target glucose range of 70–130 mg/dL before meals (i.e., fasting) and less than 180 mg/dL at the zenith of the post-meal spike. There is also some evidence that high glycemic variability (i.e., the standard deviation of blood glucose measurements) is an indicator of risk for diabetic complications independently of average blood glucose.
Accordingly, my blood glucose management goals (with respect to the data provided by the Dexcom system) are the following:
-
as many readings in the target range of 70–130 mg/dL as possible
-
keep the percentage of readings below 65 mg/dL to a minimum
-
reduce the standard deviation (as measured on a day’s worth of readings) of blood glucose readings
-
reduce the daily mean of blood glucose readings
When I started using the Dexcom continuous glucose monitoring system, my first reaction was shock. Before using the Dexcom, I had been completely (and blissfully) unaware that my post-meal blood glucose spikes were regularly above 200 mg/dL and often in the 220–230 mg/dL range, especially in the morning.
The mere fact of having a reliable feedback mechanism in the form of the Dexcom monitor was not enough to effect an improvement in my post-meal blood glucose excursions. Months passed, and I only grew more and more frustrated with my seeming inability to control my post-meal blood glucose.
The first potential solution I attempted was to try supplementing my insulin regimen with a recently-approved new drug called Symlin, but that (as they say) is a story for another time: regular use of Symlin was somewhat useful in controlling my post-meal blood glucose excursions, but in the end the effect wasn’t large enough to outweigh the considerable side effects of the drug.
A New Hypothesis
Finally, in the summer of 2011, I happened across another potential strategy for improving my blood glucose: a low-carbohydrate diet. I had been aware of the low-carb and Atkins diet fad(s), but I’d always dismissed it as nothing but a fad. It wasn’t until I read Gary Taubes’ Good Calories, Bad Calories that I really started to take a low-carbohydrate diet seriously as a viable option. Once I was convinced that carbohydrates aren’t an essential component of a healthy diet and therefore that there wasn’t any harm in trying a low-carbohydrate diet, I decided to test following a low-carbohydrate diet as a means to improving my blood glucose management.
Hypothesis: Carbohydrate restriction is an effective way to improve blood glucose outcomes.
The reasoning behind using a low-carbohydrate diet as a means to improving blood glucose outcomes is quite straightforward: the vast majority of a PWD’s blood glucose excursions (assuming his/her basal insulin has been properly adjusted) are a direct consequence of carbohydrate consumption especially given that, as mentioned above, the delivery of insulin through subcutaneous fat tissue is only a pale imitation of the insulin response in a non-PWD.
When I hear a physician saying to a type 1 diabetes patient, “Go ahead and eat whatever you want, just make sure you cover your glucose with insulin,” it’s like telling a firefighter, “Just go ahead and pour as much gasoline as you like on that fire you’re trying to put out, as long as you cover it with enough water.” Completely circular and illogical. —low-carbohydrate advocate Peter Attia, M.D., in an interview on the diabetes blog A Sweet Life
The Nitty-Gritty Details
I decided to start following a carbohydrate-restricted diet at the beginning of January, 2012. Since I already had many months of blood glucose measurements from my Dexcom system when I wasn’t following a low-carbohydrate diet, I already had the data for the control part of my experiment.
I used a variety of tools to analyze and compare the next several months of my Dexcom data, following my adoption of a carbohydrate-restricted diet:
- export files from the Dexcom software:
- XML files, useful for manipulating in Python with BeautifulSoup
- tab-delimited .csv files, useful for direct importing into R
- in R:
- built-in non-parametric statistical functions
- built-in plotting functions: boxplot(), etc.
- ggplot2 for data visualization
The Results
Statistical Significance
My first task after collecting several months worth of blood glucose data while following a carbohydrate-restricted diet was to assess whether this dietary change resulted in a statistically significant difference in blood glucose measurements. Because blood glucose data are not normally distributed (i.e., non-parametric), I used the Wilcoxon rank-sum test (similar to the more familiar Student’s t-test used with parametric data) to test the null hypothesis that the data from before and after my dietary change could have been drawn from the same population. The result came out overwhelmingly in favor of rejecting this null hypothesis, with a p-value of 2.2e-16.
I was also able to calculate an estimate of the median of the difference between a sample from my blood glucose measurements on a “normal” diet and a sample from my measurements on the carbohydrate-restricted diet, which turned out to be -19 mg/dL.
Conclusion: My adoption of a carbohydrate-restricted diet resulted in significant (negative) change in blood glucose values.
Visualizing Change
Next I turned to the task of visualizing the change in my blood glucose outcomes.
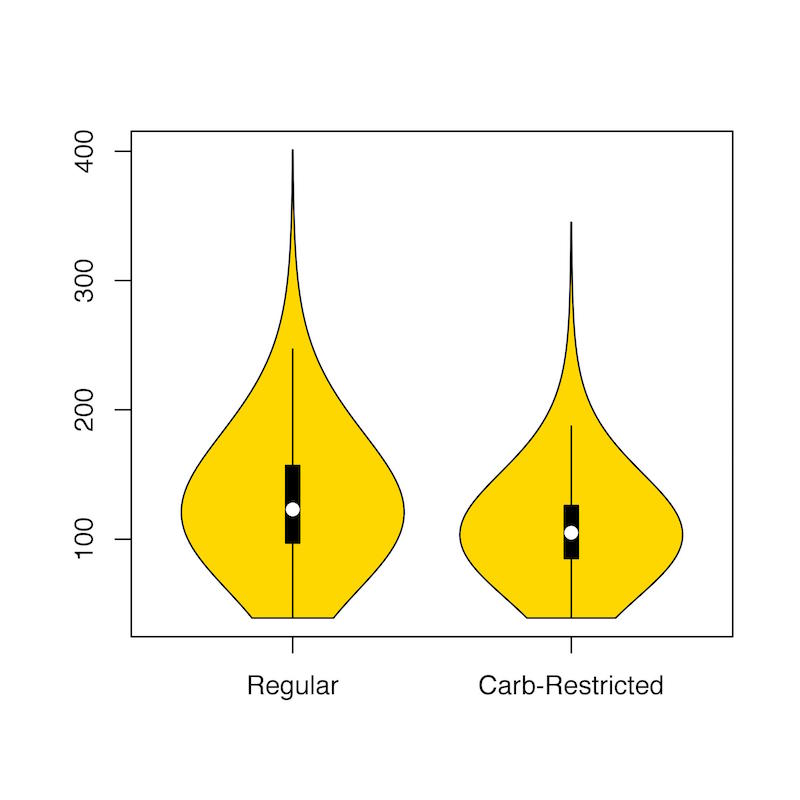
A violin plot of my blood glucose measurements from my regular diet versus the carbohydrate-restricted diet gives quite a clear picture of both the improved, lower mean glucose I achieved with a carbohydrate-restricted diet as well as the concomitant reduction in glycemic variability.
I also wanted to look at my three sub-goals for improving my blood glucose management—reducing daily mean glucose, reducing daily standard deviation, and minimizing hypoglycemic readings—each on its own. In every case, the visualizations show improvement quite clearly:
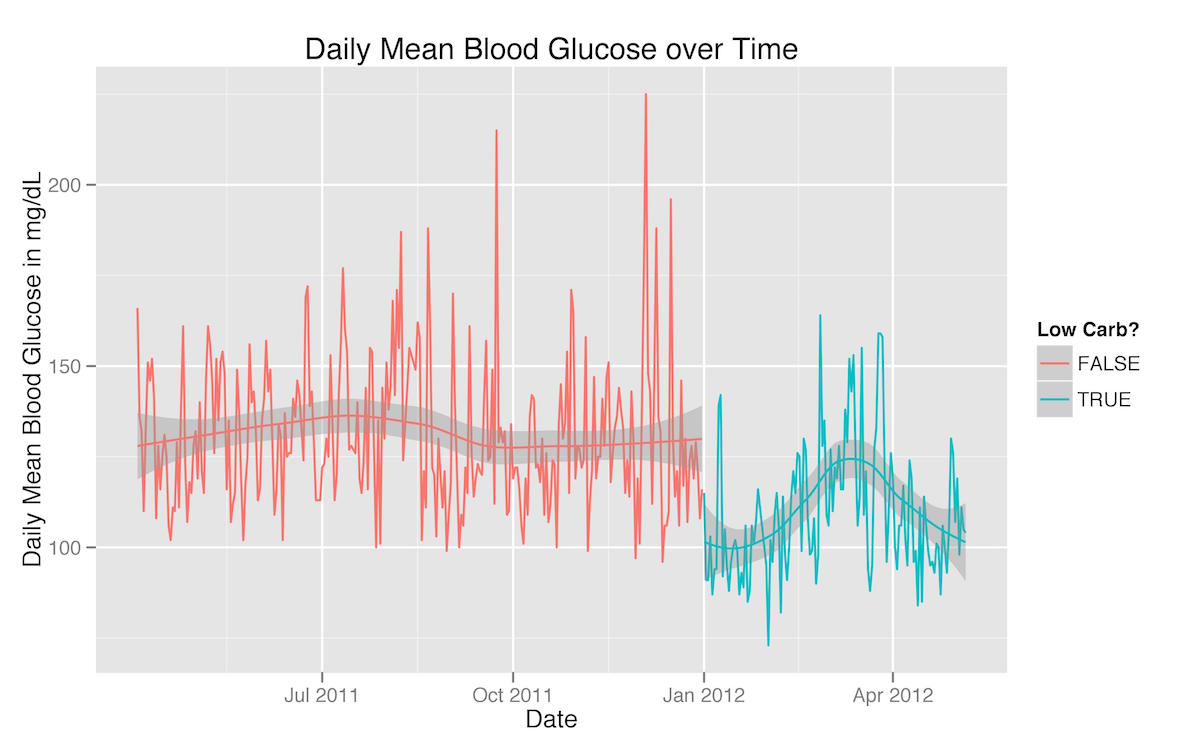
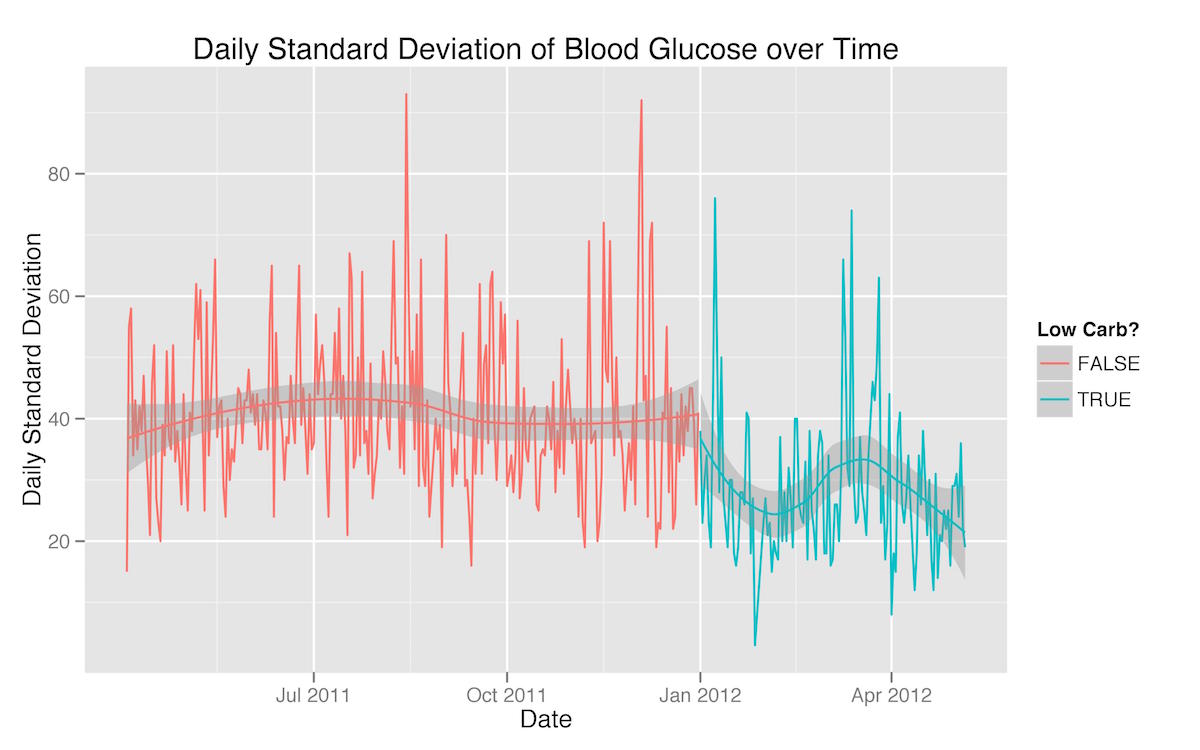
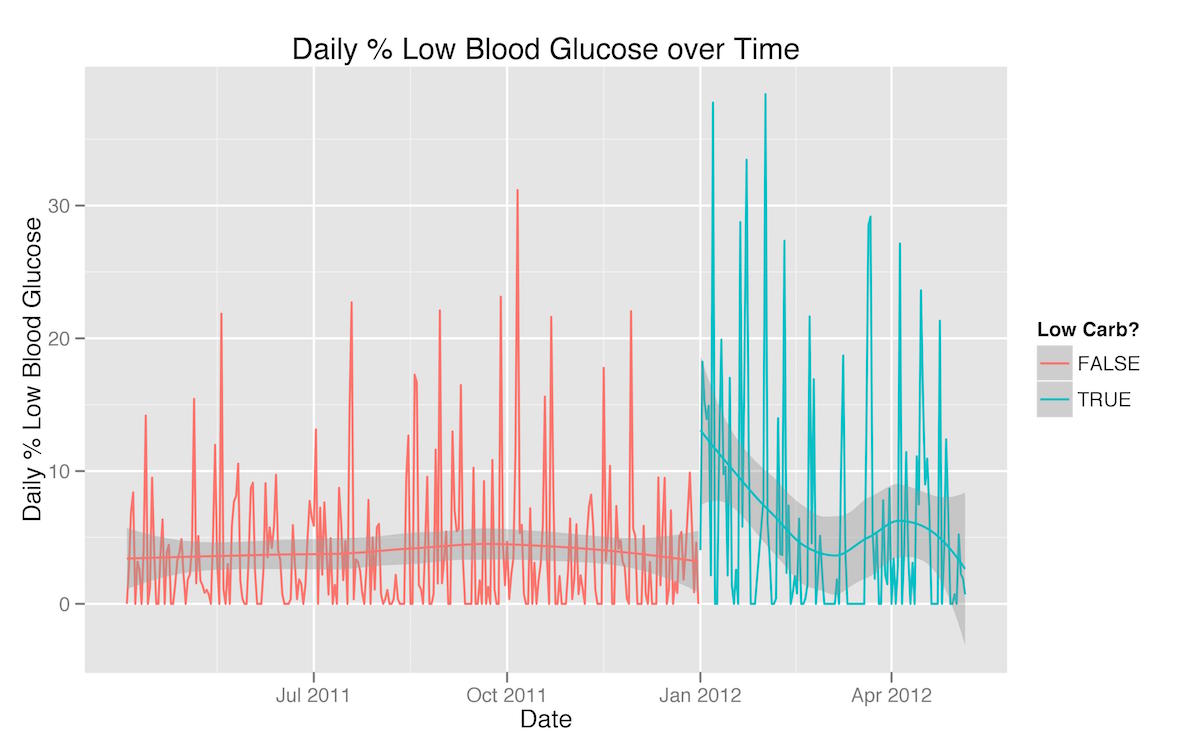
There are a few patterns evident in these three graphs that reflect extra-dietary factors that had an effect on my blood glucose management. First, the rise in both mean blood glucose and standard deviation in March is a testament to the fact that I was traveling a great deal then and found it much more difficult to adhere to the same level of carbohydrate-restriction. Second, the dramatic increase in the percentage of readings below 65 mg/dL at the beginning of my carbohydrate-restricted diet are a reflection of the fact that as soon as I started to restrict my carbohydrates, my insulin needs (basal as well as bolus) dropped significantly—in fact, faster than I was able to adjust.
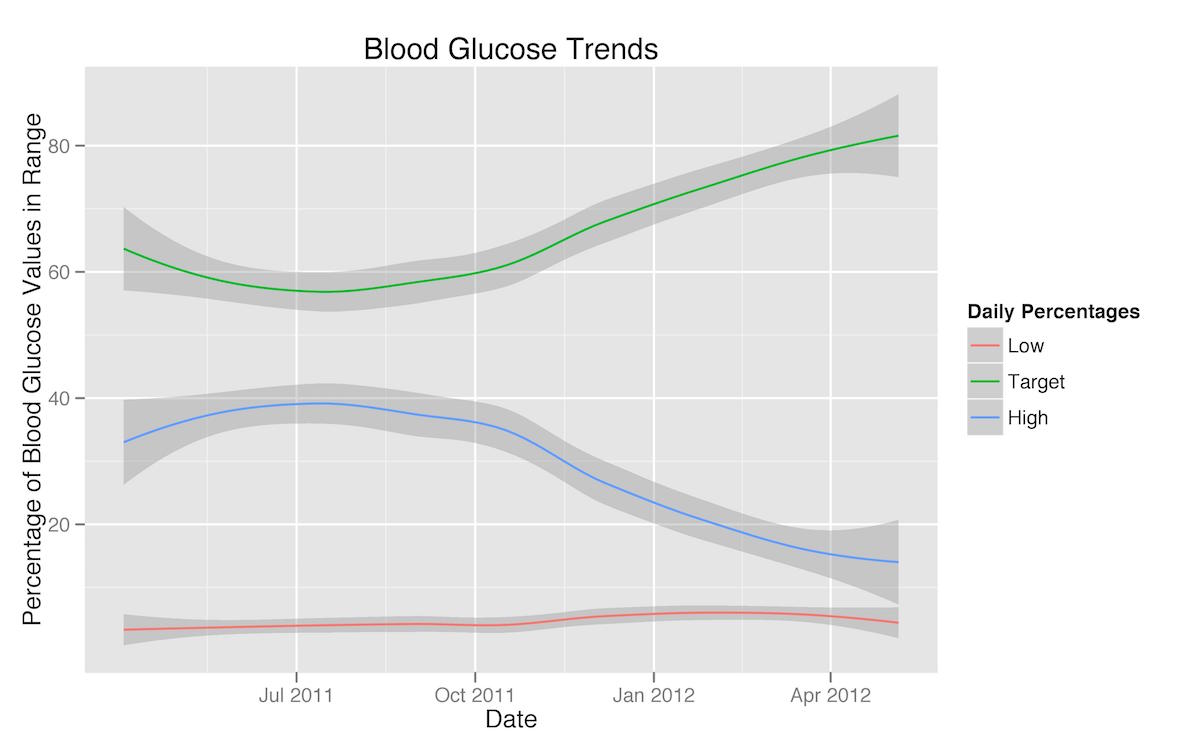
I also put together a smoothed graph of the percentages of my blood glucose readings that were hypoglycemic, in my target range, or higher than my target range. Here the trend towards a reduction in high readings and an increase in target range readings upon the adoption of a carbohydrate-restricted diet is very clear.
Finally, using a single sample week from each of the test conditions, I produced a heatmap of my blood glucose readings by time of day.
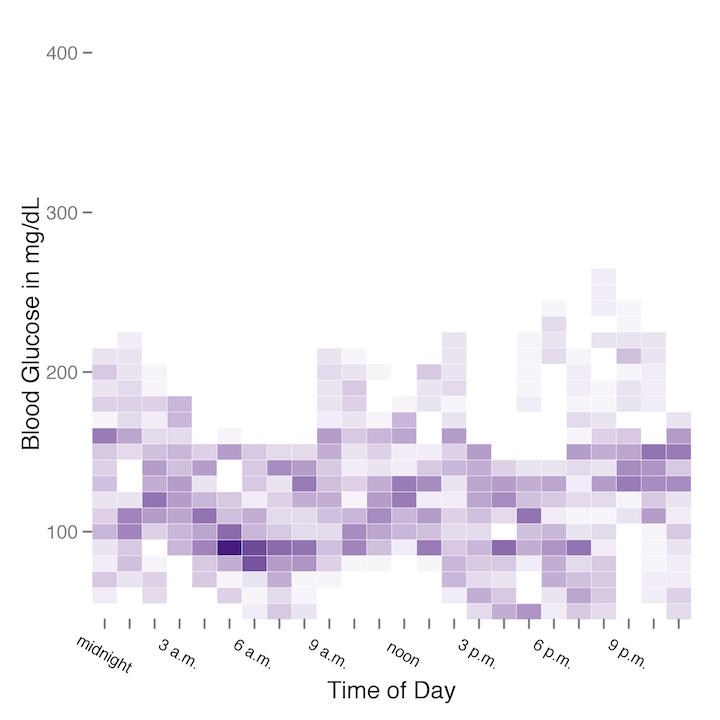
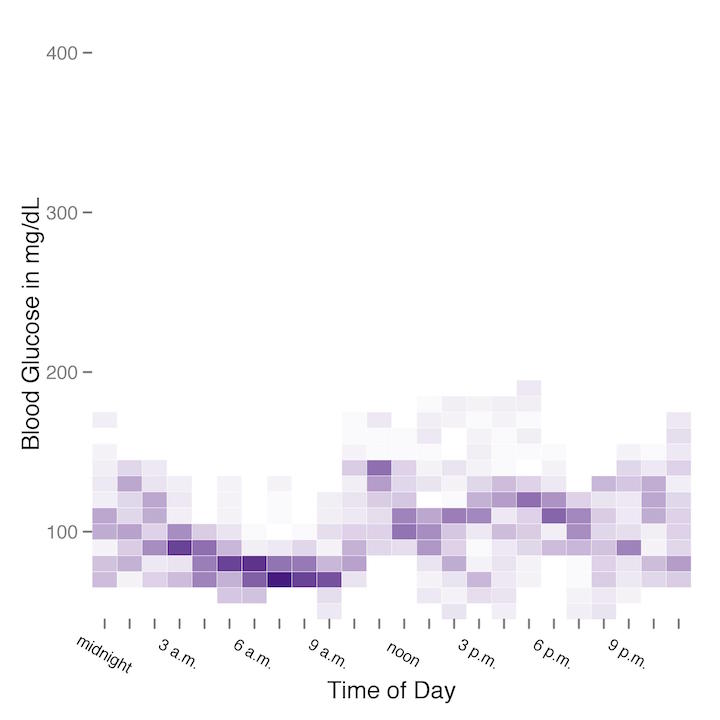
The heatmap from the regular diet week is on the left (or top), and the heatmap from the carbohydrate-restricted diet is on the right (or bottom). Although the difference between the two heatmaps is not extremely stark, it is fairly clear that glycemic variability was considerably higher on a regular diet versus a carbohydrate-restricted diet.
Looking for Patterns
By Day of the Week
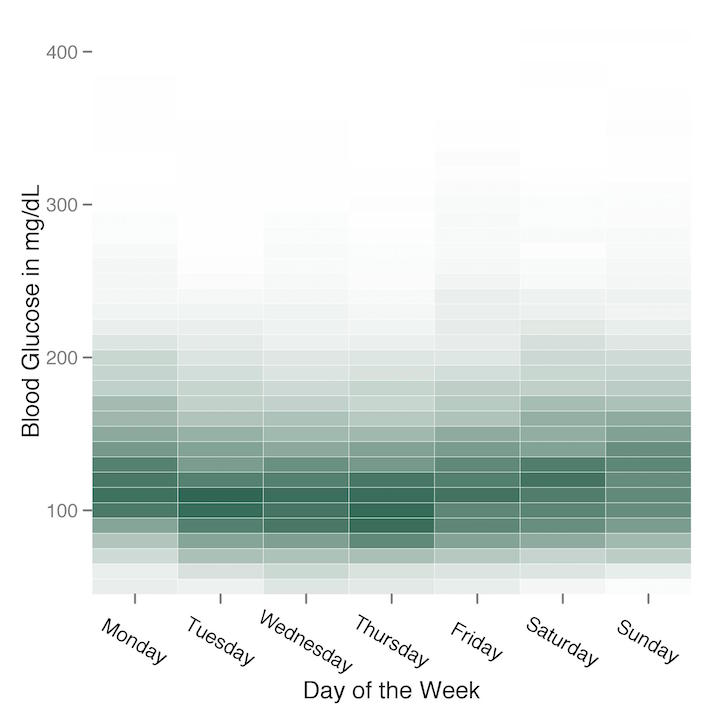
I used heatmaps again to look for whether or not there was any pattern to my blood glucose readings by day of the week. Again there doesn’t appear to be much of a pattern, although it does appear that weekends—when I’m sure I lapsed on my carbohydrate restriction more than I did during the week—show more variability than weekdays.
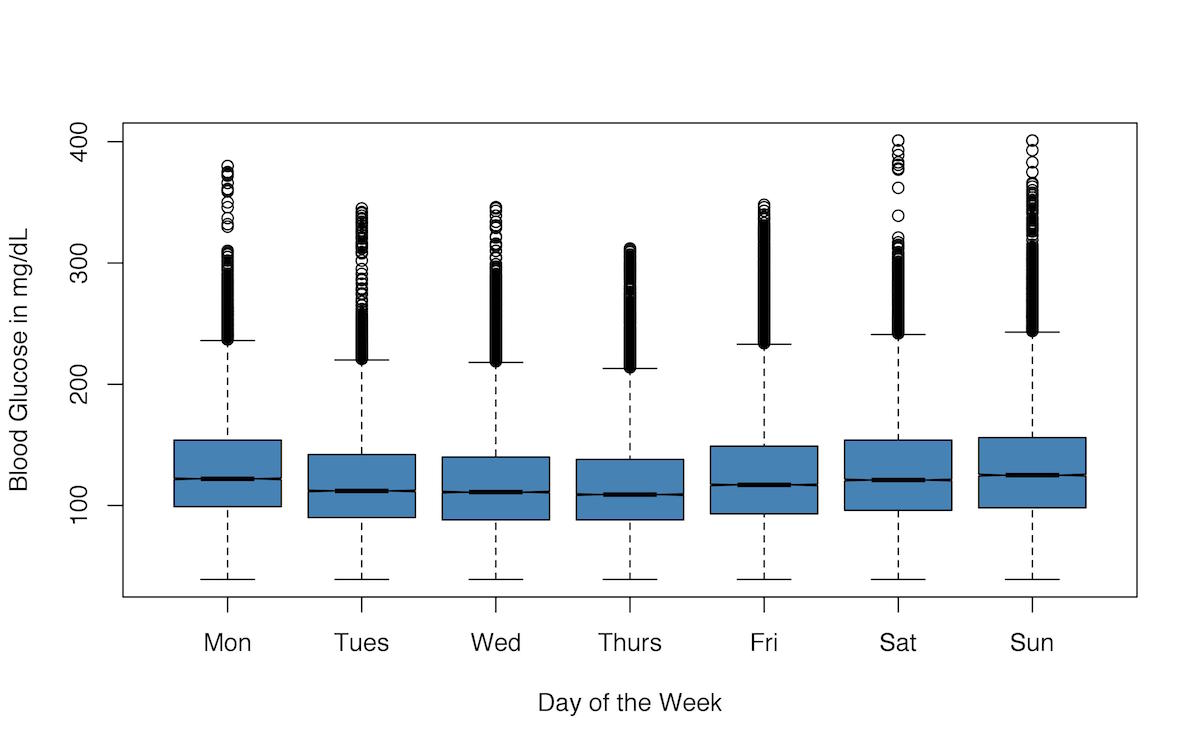
I also graphed my blood glucose readings for the different days of the week as box-and-whisker plots, but if anything, I think this visualization gives even less of an impression of a clear pattern of variance in my blood glucose readings by day of the week.
By Time of Day
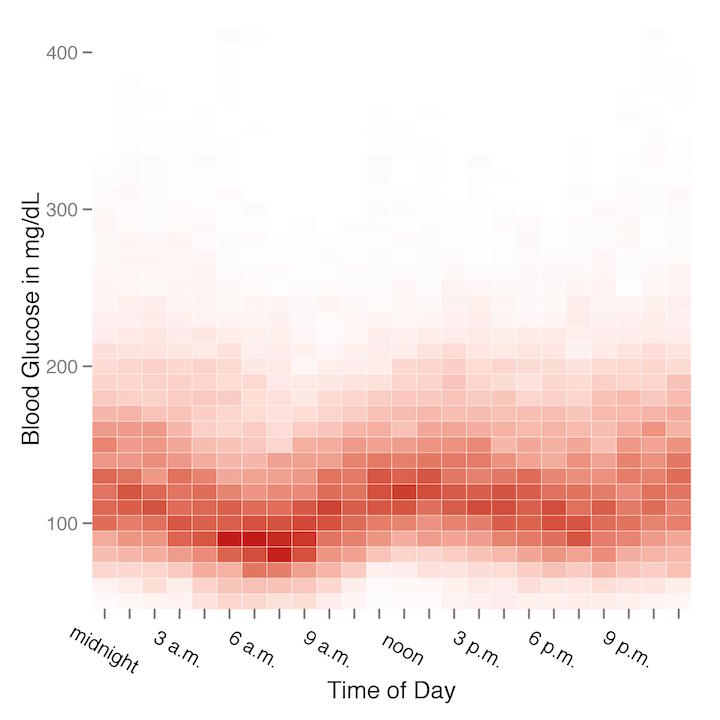
The heatmap for all my blood glucose readings by time of day is a bit more revealing. And in fact what I think this heatmap reveals most clearly is that I’m a very nocturnal graduate student! My blood glucose readings are consistently lowest between about 6 a.m. to 9 a.m., which would probably look odd to most PWDs, as many of us struggle in particular with higher blood glucose in the mornings: this is known as the “dawn phenomenon.” It’s not that I’m an unusual PWD who doesn’t struggle with the dawn phenomenon; rather, my dawn phenomenon just happens a few hours later than usual, peaking in the early afternoon hours instead of the morning because of my habitual late nights and late mornings.